Systems Biology of Retrotransposition
Mechanisms, Impact, and Role in Aging
We investigate retrotransposons — primarily LINE-1 (L1), an autonomous element, with expanding focus on non-autonomous elements such as Alu and SVA — within mouse and human genomes. These elements actively propagate, forming complex networked systems that interact with cells at multiple levels:
Key Focus Areas
1. Mechanism of Replication
Retrotransposons use host cell machinery to replicate their genome and integrate into host DNA.
2. Targeting and Insertion Preferences
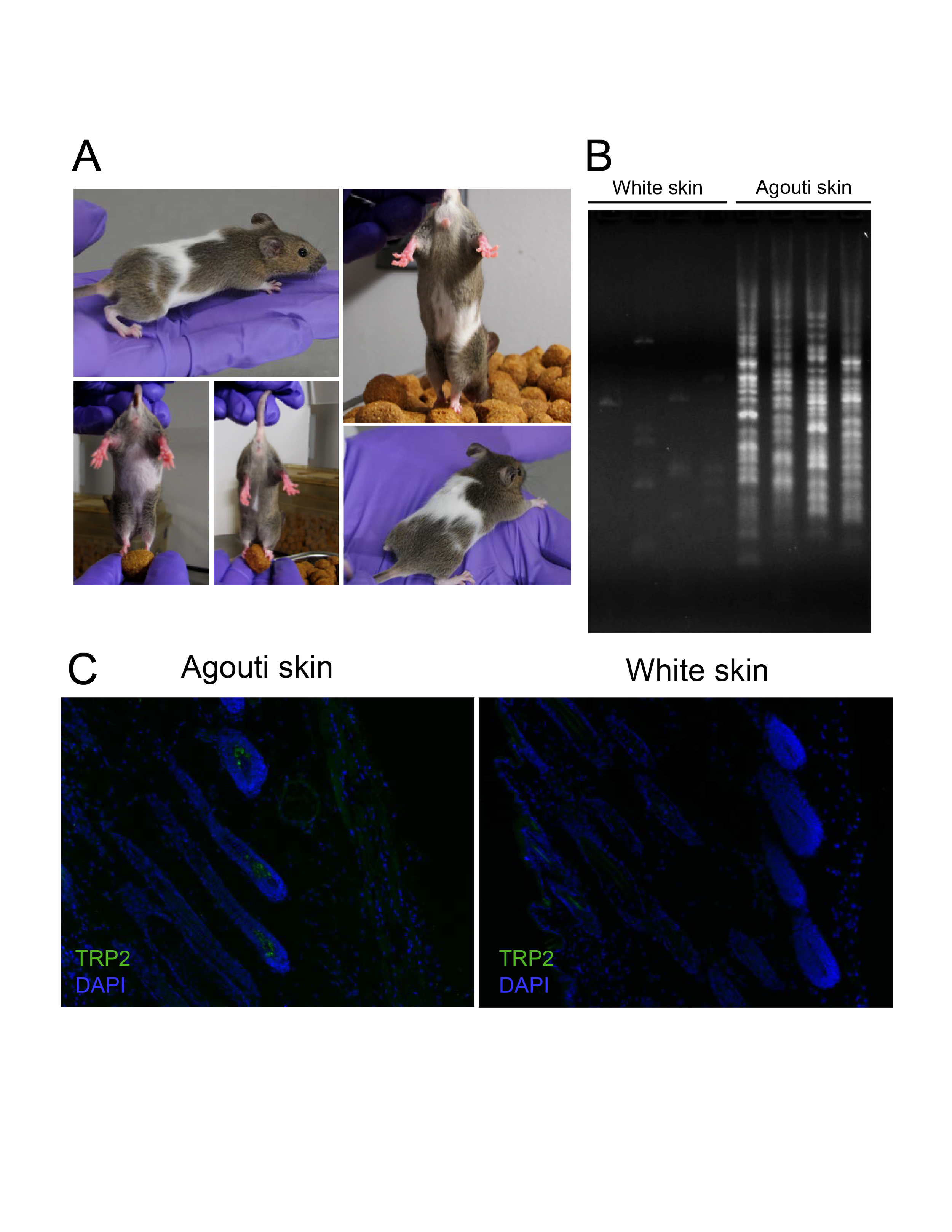
Each type of transposable element exhibits preferences for specific DNA regions. Factors like DNA sequence, chromatin structure, host protein interactions, and 3D nuclear positioning may influence insertion site selection.
3. Cellular and Tumor-Type Impact
Insertion frequency varies depending on cellular environments, with LINE-1 insertions more prevalent in certain cancers, such as colon cancer. Why do some cells resist transposition better than others?
4. Host Co-evolution
Retrotransposons act as molecular "parasites," triggering evolutionary adaptations in hosts. We aim to uncover how organisms respond to newly introduced retrotransposons and identify genes under positive selection that enhance defenses against these elements.
Retrotransposons and Aging
In young, healthy cells, retrotransposon activity is tightly repressed by epigenetic mechanisms, including DNA methylation, histone modifications, and RNA interference. However, during aging, these silencing systems weaken, resulting in elevated retrotransposon activity across various species — from yeast and flies to mice and humans.
Reactivation of retrotransposons contributes to hallmark features of aging, such as:
Genomic Instability: Insertional mutagenesis, DNA breaks, and replication stress.
Inflammaging: Retrotransposon-derived RNAs and cDNAs activate innate immune responses, promoting chronic inflammation, a key driver of aging-related decline.
Cellular Senescence: Increased LINE-1 expression is linked to age-associated diseases and functional deterioration.
Experimental approaches to reinforce retrotransposon repression, such as enhancing heterochromatin structure or RNA interference, have demonstrated lifespan extension in model organisms. Conversely, increased retrotransposon activity accelerates aging phenotypes. As a result, retrotransposons are being explored not only as contributors to aging pathology but also as biomarkers of declining epigenetic integrity.
O'Donnell KA, An W, Schrum CT, Wheelan SJ, Boeke JD. Controlled insertional mutagenesis using a LINE-1 (ORFeus) gene-trap mouse model. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):E2706-13. doi: 10.1073/pnas.1302504110. Epub 2013 Jul 1. PMID: 23818630; PMCID: PMC3718180.
Our Approach
We are a multidisciplinary team combining experimental, informational, and computational sciences to explore transposable elements and their interplay with host biology. By decoding the complexities of retrotransposon-host interactions, our research holds promising applications — from gene therapy and forward genetic screens to understanding their roles as somatic mutagens and inherited variants in human disease.
Recognizing the broader relevance of transposable elements, we actively collaborate with the larger scientific community to advance research, foster innovation, and deepen insights into these fascinating genomic elements.
Support
Role of Retrotransposon Activity in Neurodegeneration and Alzheimer's Disease.
NIA 5P01AG051449
Principle Investigator: J. Sedivy.